
Vivani™ is an innovative,
near-clinical stage biopharmaceutical company.
Leveraging our proprietary NanoPortal™ platform technology, we are developing a portfolio of miniature, long-term drug implants to address two main barriers of optimal chronic disease treatment: medication non-adherence and tolerability. Our lead assets, NPM-115 and NPM-119, are miniature, six-month, GLP-1 (exenatide) implants under development for chronic weight management/obesity and type 2 diabetes, respectively.
We seek to maximize the benefit and minimize the burden of treatment for patients with chronic conditions.
We believe our growing portfolio could help patients live free from the administration hassles of daily or weekly medications. For physicians, caregivers and loved ones, this would mean greater confidence that patients are receiving their prescribed doses and potentially achieving better clinical outcomes.

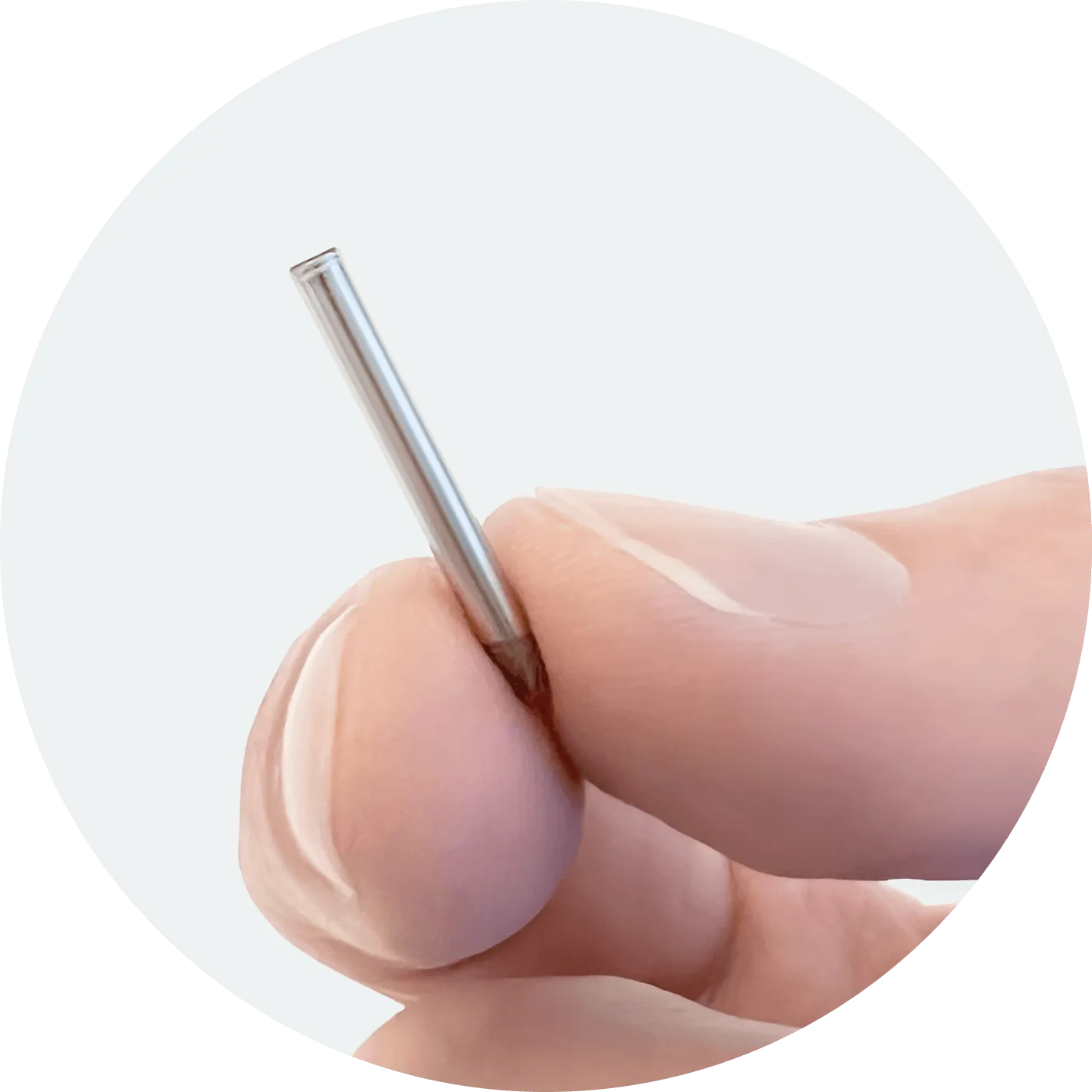
Developing long-term drug implants with the potential to guarantee medication adherence and improve tolerability.
NPM-115 is a pharmaceutical GLP-1 agonist (high-dose exenatide) implant under investigation for chronic weight management in obese or overweight individuals.
NPM-119 is a pharmaceutical GLP-1 agonist (exenatide) implant under investigation for the treatment of patients with type 2 diabetes.
Positioning Vivani for
pipeline execution.
We strive to build the future of Vivani through both a strong capital base and the expertise of our entrepreneurial innovators. Our scientists and executives pool their collective knowledge in drug development, implant technology and chronic diseases to drive our pipeline forward.

Building a revolutionary machine-to-human interface.
Cortigent is a subsidiary of Vivani developing targeted neurostimulation systems to help patients recover critical body functions and regain control of their lives. Their technology combines innovative neuroscience with proprietary microelectronics, software, and data processing capabilities to provide artificial vision and potentially restore muscle movement during stroke rehabilitation.
Cortigent is advancing the pioneering work of legacy company, Second Sight Medical Products, whose revolutionary product, the Argus II® Retinal Prosthesis System, is the world’s first and only device authorized by the FDA to provided artificial vision to profoundly blind people. For more information, email info@cortigent.com.