Guaranteed adherence. Better outcomes.™
Through miniaturized, long-term biopharmaceutical implants, we seek to free patients from medication adherence challenges, minimizing the burden and maximizing the benefit of clinical treatments.
Addressing the biggest challenges in chronic disease treatment – medication non-adherence and tolerability.
An alarming percentage of patients suffering from chronic illnesses don’t take their medications as prescribed, often due to the inconveniences associated with drug administration and/or tolerability, which may be associated with fluctuating drug levels.
Watch our CEO, Dr. Adam Mendelsohn, introduce our revolutionary approach to improve chronic disease treatment with miniature, ultra long-acting drug implants.Our long-term solution for life-long conditions.
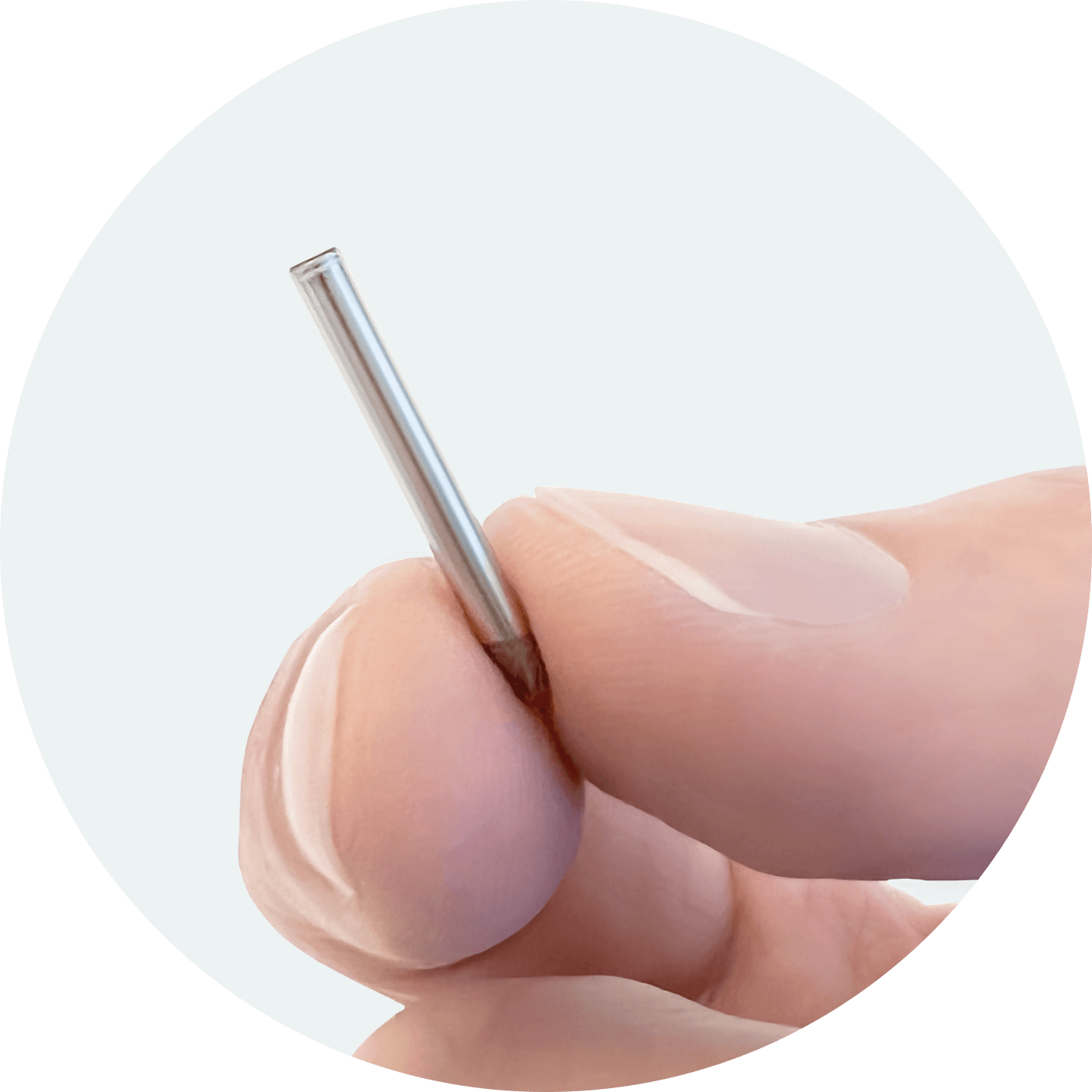
Our Lead Drug Candidate: NPM-139
GLP-1 for Chronic Weight Management
NPM-139 is a miniature, subdermal, semaglutide drug implant with once or twice-yearly dosing under development for chronic weight management in patients who are obese or overweight. This implant can be conveniently inserted under the skin during a quick outpatient procedure and is designed to provide steady doses of medication. Based on positive preclinical weight loss data, NPM-139 is now planned for clinical development. NPM-115, a miniature, six-month, subdermal, subdermal, exenatide implant, was the first NanoPortal implant to achieve 6-month feasibility in animals and was used in LIBERATE-1, the Phase 1, first-in-human application of the company’s proprietary NanoPortal implant technology. LIBERATE-1 achieved all primary objectives and has paved the way for future development of the technology with our emerging pipeline.
Also Developing Candidate: NPM-133
GLP-1 for Type 2 Diabetes
NPM-133 is a miniature, six-month, subdermal, semaglutide drug implant under development to treat patients with type 2 diabetes. This device can also be conveniently inserted under the skin during a quick outpatient procedure and is designed to provide steady doses of medication for up to six months.

