
Revolutionizing the treatment of chronic disease.
Leveraging our NanoPortal™ technology, we are developing a portfolio of highly differentiated products comprised of miniature, sub-dermal drug implants.
Our Pipeline
high-dose exenatide
exenatide
semaglutide
exenatide
high-dose exenatide
exenatide
semaglutide
exenatide
high-dose exenatide
exenatide
semaglutide
exenatide
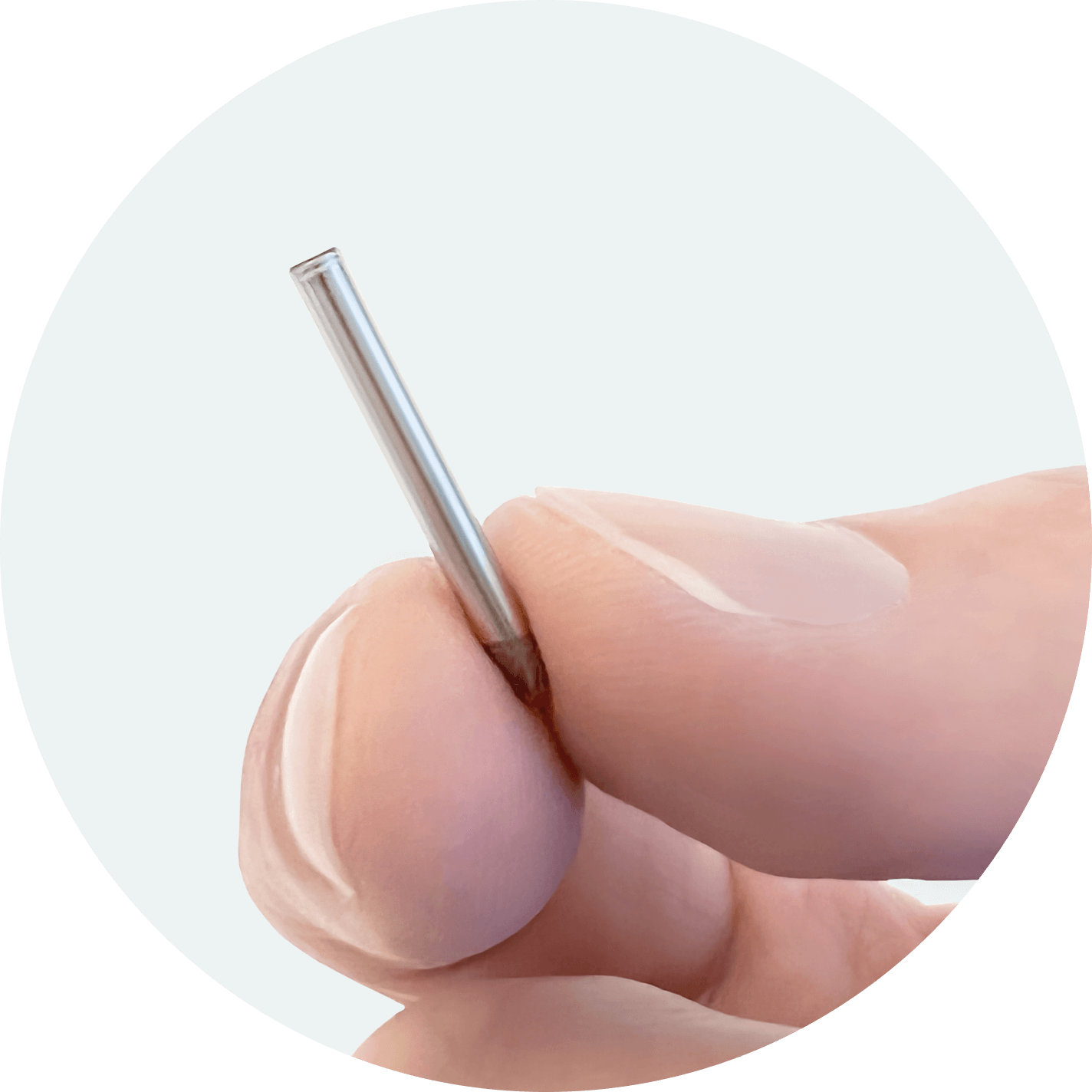
NPM-115 Overview
A miniature, GLP-1 receptor agonist implant designed to address medication non-adherence and improve tolerability.
NPM-115, leveraging our NanoPortal™ technology, is designed to provide steady, long-term therapeutic delivery of high-dose exenatide for at least six months.
By assuring medication adherence, NPM-115 may free patients with obesity from burdens associated with oral and injectable medications, as well as provide confidence to physicians, caregivers and loved ones that patients are receiving the intended therapeutic benefits from their medicine.
Target
Under investigation for chronic weight management/obesity.
Although the GLP-1 receptor agonist class has quickly outpaced previous anti-obesity medications due to superior efficacy and tolerability, medication non-adherence continues to affect an alarming number of patients – approximately 50%, including those taking daily pills. In fact, non-adherence may also contribute to the gap in real-world effectiveness compared to the efficacy reported from randomized, controlled, clinical trials.
Development
First-in-Human clinical trial.
Our first-in-human clinical study, called LIBERATE-1, is ongoing and was initiated in December 2024 at two study centers in Australia.
LIBERATE-1 is the first clinical study investigating the effects of our GLP-1 implant in obese or overweight individuals and the first clinical study utilizing our platform NanoPortal implant technology.
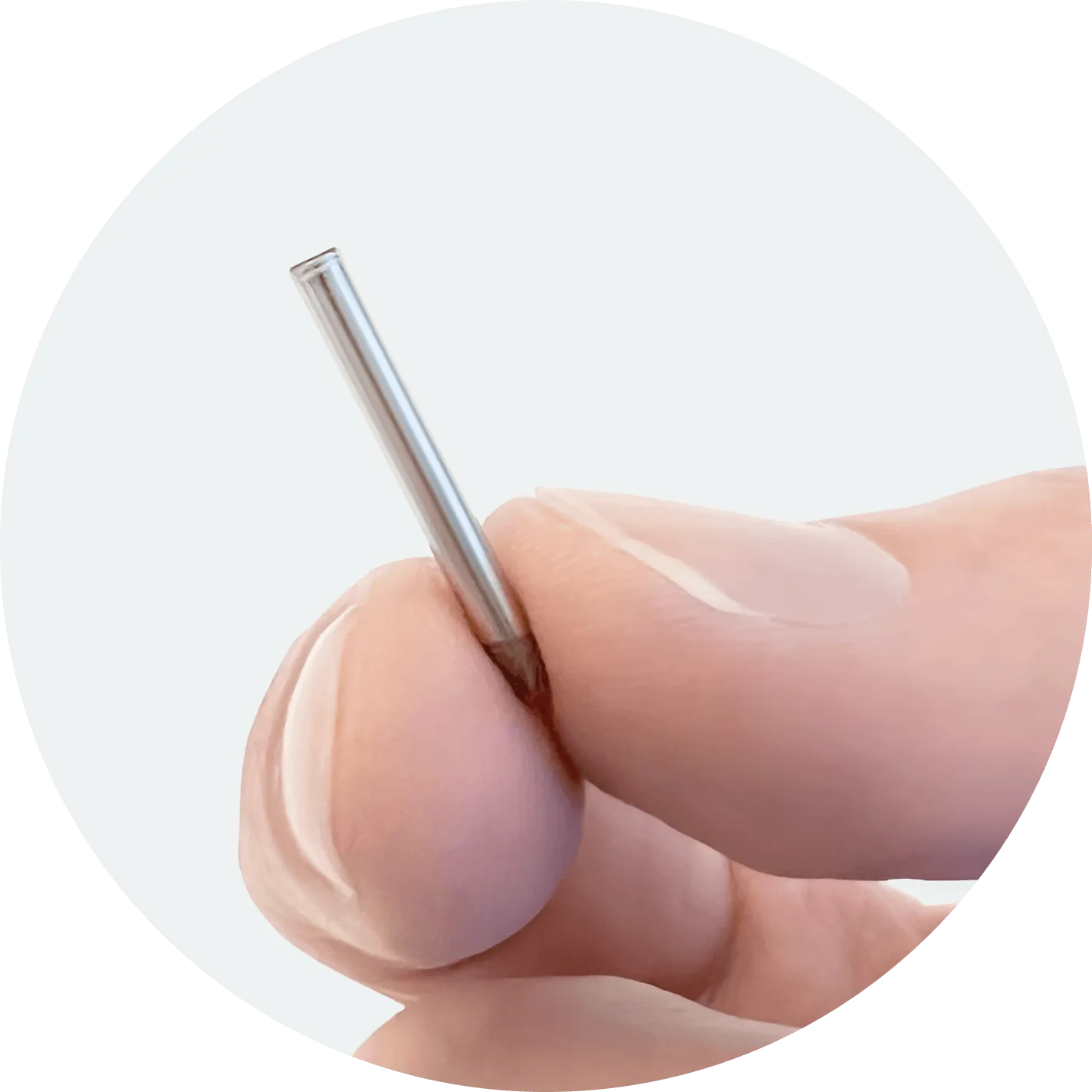
NPM-119 Overview
A miniature, GLP-1 receptor agonist implant designed to address medication non-adherence and improve tolerability.
Built with our NanoPortal™ technology, NPM-119 is designed to provide steady, long-term therapeutic delivery of exenatide for at least six months.
By assuring medication adherence, NPM-119 may free patients with type 2 diabetes from burdens associated with oral and injectable medications, as well as provide confidence to physicians, caregivers and loved ones that patients are receiving the intended therapeutic benefits from their medicine.
Target
Under investigation for the treatment of type 2 diabetes.
Medication non-adherence affects an alarming number of patients – approximately 50%
Development
Portfolio Synergies.
In addition to providing support for NPM-115, our lead program in weight management, the LIBERATE-1 trial will also provide key insight for the NPM-119 program by including a Bydureon (exenatide injection) arm enabling pharmacokinetic comparison of exenatide concentrations between the exenatide implant and the marketed product. This information will help assess the translation of exenatide delivery in animal models to exenatide delivery in humans.